
As licensees and proprietors prepare for the new Standards for the Operation of Licensed Pharmacies (SOLP) to come into effect on February 1, 2025, there are a number of key changes to consider. Following is a list of some significant changes.
- New structure: Six domains
The new SOLP are categorized into six domains critical to effective pharmacy operations. The domain of person-centred care is foundational to every aspect of pharmacy practice and, by extension, pharmacy operations. Similarly, the domain of professionalism and leadership is fundamental to all other domains as it is an intrinsic part of all activities that licensees and proprietors perform.
Each domain is divided into topics. Each topic has an outcome standard which describes the expected patient outcome that must be achieved for the standard to be met. The achievement of each outcome standard is detailed further through descriptive standards that provide specific details about the activities important to achieving the required outcome.
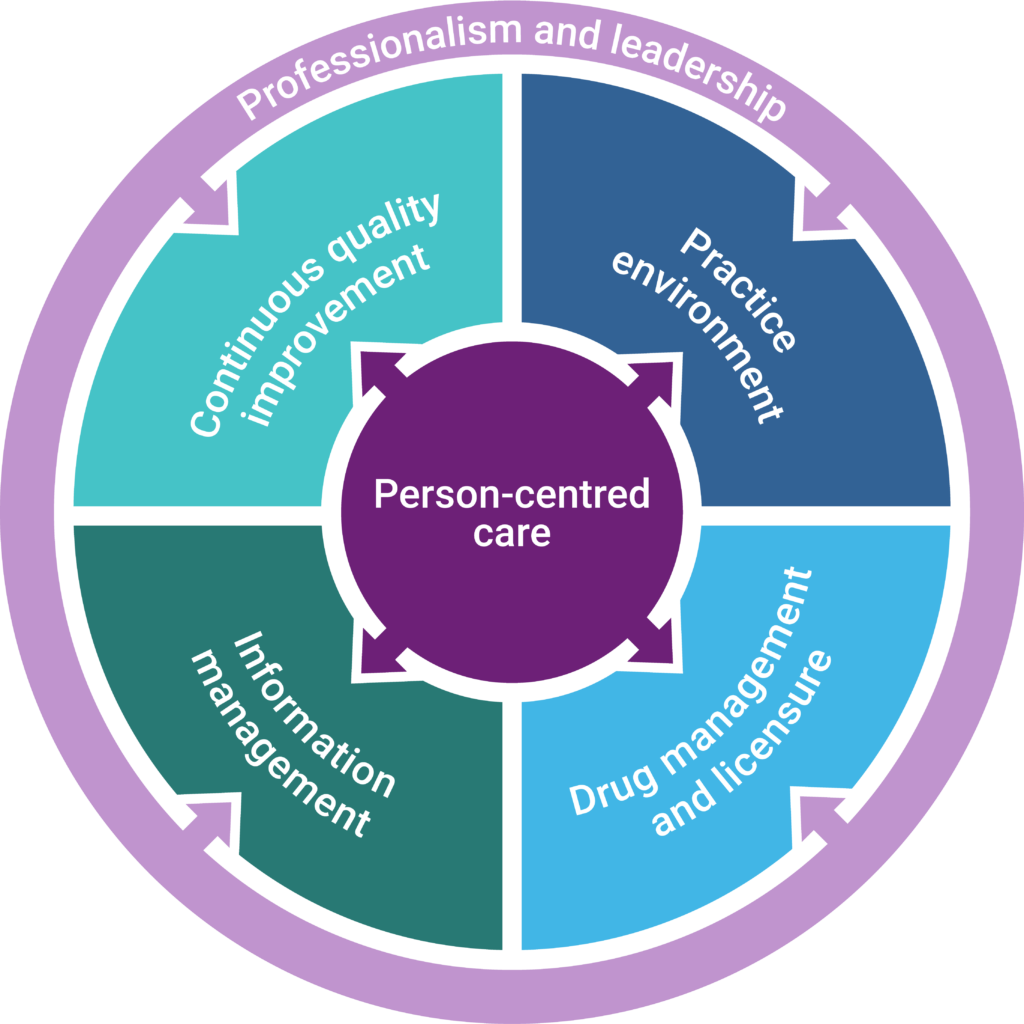
- Person-centredness sets the foundation (Domain 1)
This concept has been expanded on in the new SOLP. Although these standards are operational in nature, wherever possible, the standards correlate to a patient-centred outcome and require the proprietor’s representative and licensee to maintain a practice environment that enables regulated members to provide person-centred care.
- Proprietors prohibited from imposing conditions or quotas (Standard 2.1.2)
Current standards prohibit proprietors from imposing conditions that compromise the professional judgement of licensees and regulated members. The new standards clarify that this prohibition includes imposing quotas that require licensees to achieve any measurement of professional services or revenue obtained from professional services provided to patients.
- Defining a licensee’s responsibilities when their pharmacy’s regulated members work remotely (Standard 2.10)
It is increasingly common for regulated members to provide care from a licensed pharmacy while working remotely outside of the pharmacy. Standard 2.10 outlines requirements when a member of the pharmacy team practises at a location offsite from a licensed pharmacy.
- Licensees’ oversight of other regulated health professionals (Standard 2.11)
The new standards provide guidance for licensees who have non-pharmacy regulated health professionals working as part of their pharmacy team. The new SOLP describe the requirements to enable this practice and differentiate this situation from those in which other regulated health professionals are practising independently in a shared premises with the licensed pharmacy.
- Introducing the concept of an operations supervisor (Standard 2.12)
The concept of an operations supervisor was added to the new standards to enable licensees to delegate administrative, managerial, and operational duties to a pharmacy technician or pharmacist designated as an operations supervisor.
- Ensuring appropriate supervision of unregulated employees (Standard 2.13)
The new SOLP provide a more comprehensive outline of the role and limitations of unregulated employees working in a licensed pharmacy, including pharmacy assistants. The concepts of critical steps and independent double checks are introduced to enhance patient safety and to clarify the level of supervision required for unregulated employees.
- Licensee and proprietor roles in creating a safe and professional work environment (Standard 3.1)
The new standards address appropriate staffing and employee well-being to support the provision of quality care while at the same time ensuring a safe environment for pharmacy team members.
- Clarifying requirements for the physical specifications of a dispensary (Standard 3.3.1)
The new SOLP clarify that the required minimum 18 m2 area of a dispensary does not include secondary dispensary areas that are separated by public space or separate rooms that are dedicated to compounding. New pharmacies will have to comply immediately before becoming licensed. Licensees and proprietors for existing pharmacies whose area does not comply with the standards will be required to make necessary changes the next time the pharmacy applies for a renovation.
- Displaying and advertising drugs in relation to homeopathic products (Standard 4.1.2(d))
The new SOLP require that drugs are displayed and advertised independently of homeopathic products by means of physical separation or signage such that a member of the public can easily distinguish drugs in the pharmacy from any homeopathic products.
- New requirements for compounding services (Standard 4.8.1)
The requirement for pharmacies to perform at least level A compounding in-house has been removed. The new SOLP require that all licensed pharmacies must provide patients access to compounding services, but pharmacies may meet their obligation by either performing compounding directly in the pharmacy in accordance with the compounding standards, or through an agreement with a pharmacy located in Alberta that holds a compounding and repackaging licence.
- Real-time transmission of data to the Alberta Netcare Electronic Health Record now required (Standard 5.3.1(b))
The new standards require that dispensed drugs for patients be submitted to and be accessible in Netcare. Further, the standard requires that this submission of data must occur in real time. Licensees of community pharmacies will have until July 1, 2026, to ensure their pharmacy transmits data to Netcare in real time. An exception is made for compounding and repackaging pharmacies that do not have a community licence.
Note that this is not a comprehensive list of changes. Licensees and proprietors must review the new SOLP in their entirety to ensure preparedness and readiness to comply prior to February 1, 2025, in most instances.
For more information on the new SOLP, refer to the standards page.




