


The reporting, investigation, and analysis of medication incidents are important elements in improving patient safety, but these efforts must be accompanied by effective strategies that address the contributing factors or vulnerabilities leading to the incidents. In turn, improvement strategies enable further preventative actions.
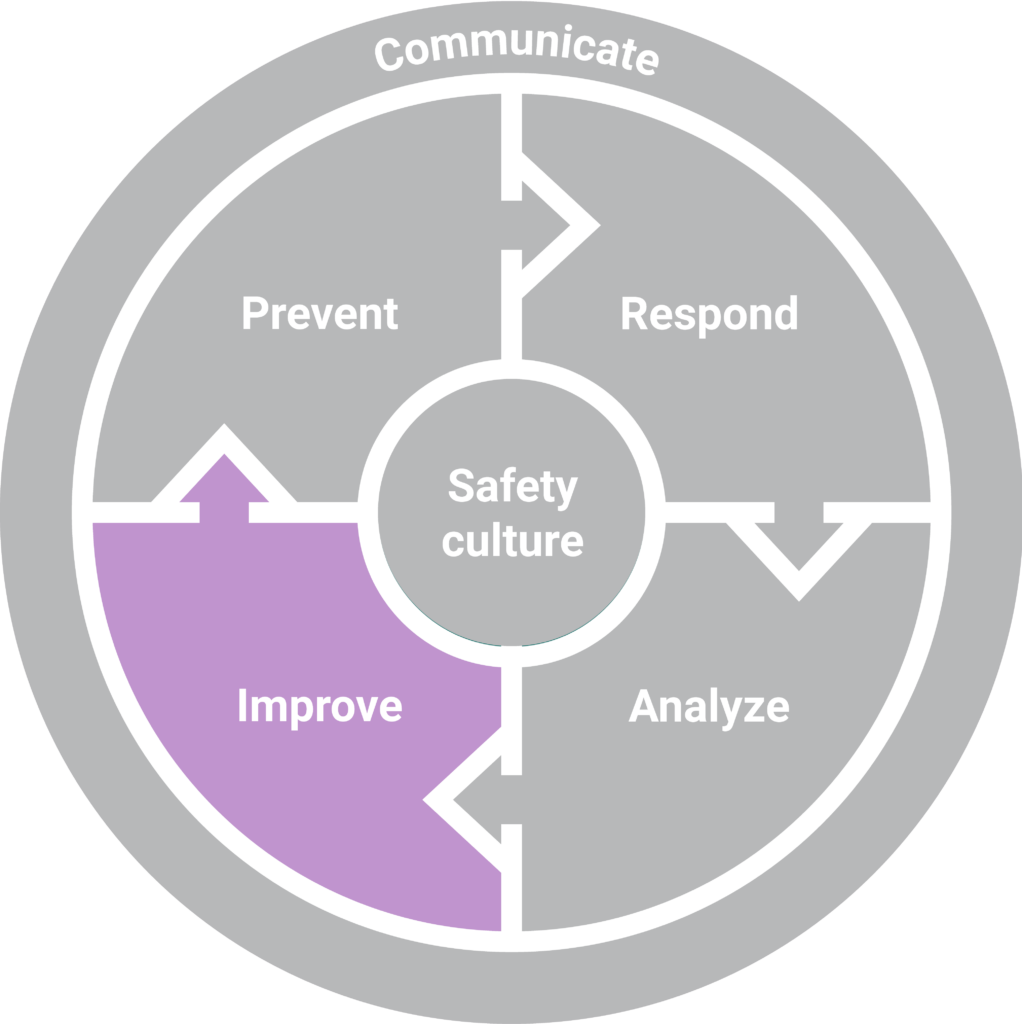
Licensees must work with the pharmacy team to identify and document action plans in their practice incident management platform to address the risks identified.
Certain types of risk-mitigation strategies have been shown to be more effective than others. In general, system-based approaches yield the most effective results, while person-based approaches can be least effective. With that said, system-based strategies are often the most complex – and resource intensive – to implement. In contrast, person-based strategies can usually be implemented quickly and with minimal impact on resources.2 Pharmacy teams will be faced with the challenge of identifying opportunities for improvement that will have a tangible impact on safety while balancing the practicality of the proposed solutions. Pharmacy teams can consider implementing several strategies simultaneously, both system-based and person-based, to achieve maximum effectiveness.
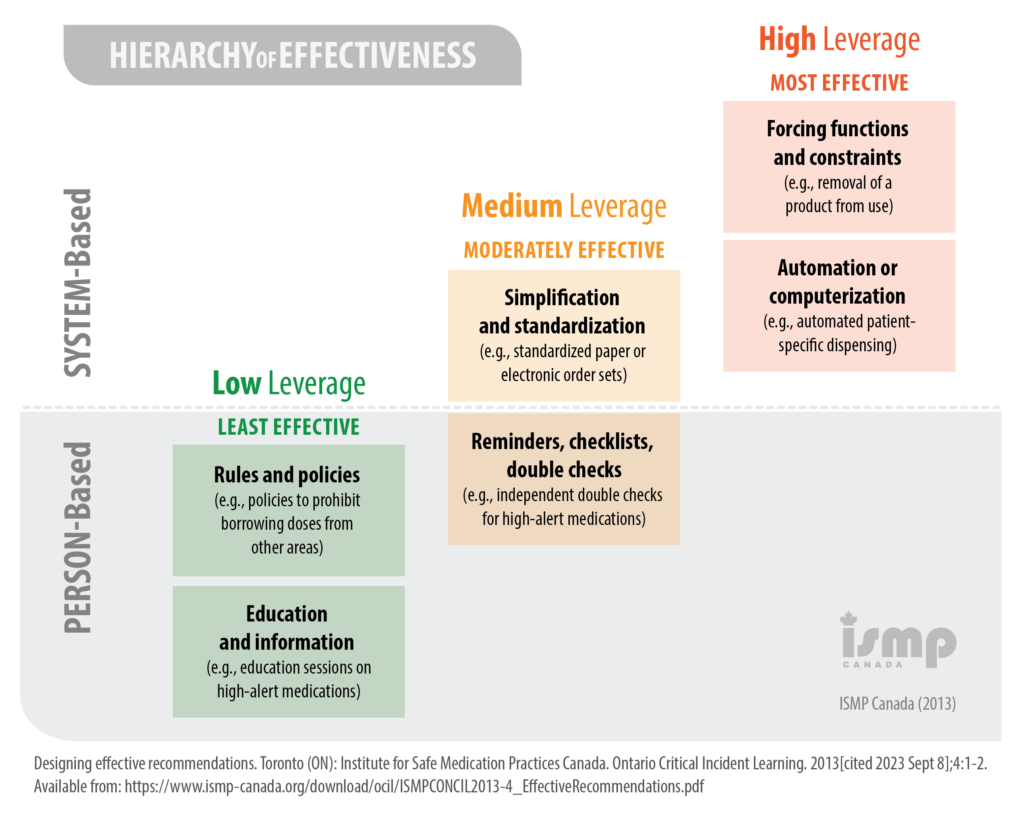
When developing action plans for improvement, pharmacy teams must ensure their plans are SMART (Specific, Measurable, Attainable, Relevant, Time-bound).
The pharmacy team agrees that addressing the storage of the refrigerated products is a priority. While this is likely a medium-leverage change, it is simple and quick to implement. Jenna remarks that this strategy could be applied to other look-alike drugs in the fridge. The team works to re-organize the fridge inventory so look-alike products are kept apart, and are stored within dedicated, well-labelled baskets. Not only will this strategy seek to prevent recurrence of the insulin incident, it will prevent occurrence of other incidents related to look-alike refrigerated drugs.
Farida calls the Shevchenkos and suggests a meeting to discuss the outcome of the pharmacy team’s analysis of the practice incident. They agree and come into the pharmacy the next day to meet with Farida and Yan, the licensee. The Shevchenkos are appreciative that the pharmacy team has taken the practice incident seriously, and agree with the suggested course of action. Farida also asks if they have any ideas for improvement, at which time Mr. Shevchenko suggests that the pharmacy team show him his medications when he picks them up, so everyone can be sure they’re correct.
After the meeting, Yan logs into the pharmacy’s practice incident management platform and updates the “Actions taken at pharmacy level” section, using the SMART format:
Right away, we will organize the fridge to ensure all insulin products are stored in distinct bins, not in close proximity to look-alike products. Within a week, we will update our policies and procedures to reflect the new storage system for refrigerated products and ensure all staff are trained on the new procedures. We will seek to have zero practice incidents related to look-alike refrigerated products moving forward.
Yan enters additional actions taken and completes the report. He checks it over one last time to ensure no individually identifying information is contained within fields that will be submitted to the national database. Once he’s satisfied with his documentation, Yan submits the report, and it is automatically transmitted securely to the NIDR by the practice incident management platform.
The team uses their next safety huddle to discuss and reinforce the importance of adhering to their existing policies around barcode scanning and showing patients their medications at the pickup counter. Yan congratulates the team on working together to analyze this practice incident and make the pharmacy environment safer.
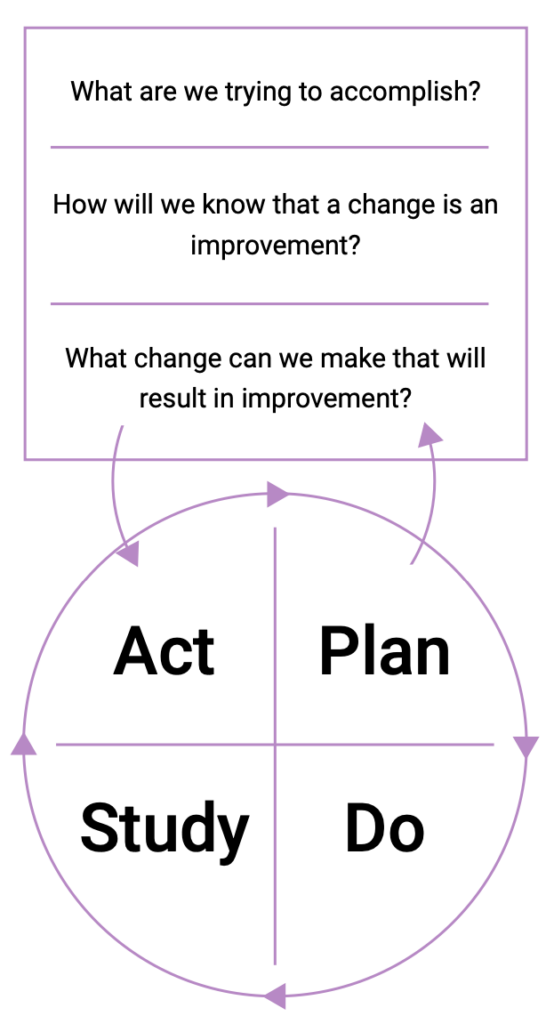
The pharmacy team’s safety journey doesn’t end with implementing change. Opportunities for learning remain, and they can be identified through continuous evaluation of the new processes that have been implemented. One tool that can be used to support the continuous evaluation of change is the Plan-Do-Study-Act (PDSA) cycle.3
The PDSA cycle is a useful tool for evaluating change. To conduct a PDSA cycle, develop a plan to test the change (Plan), carry out the test (Do), observe, analyse, and learn from the test (Study), and determine what modifications, if any, to make for the next cycle (Act).
In a healthy safety culture, the PDSA cycle continues as the pharmacy team continues to evolve its policies, processes, and systems to make care safer and more effective.
Incorporate PDSA into the pharmacy’s CQI meetings and huddles.
View information about registrants suspended or cancelled in relation to unprofessional conduct based, in whole or in part, on sexual abuse or sexual misconduct.
The information in this register is updated once every 24 hours. For more information, please review our disclaimer.